The Therapeutic Potential of Amniotic fluid-derived Stem Cells on Busulfan-Induced Azoospermia
- kübra:)

- Mar 14, 2021
- 2 min read
The Therapeutic Potential of Amniotic fluid-derived Stem Cells on Busulfan-Induced Azoospermia in Adult Rats
Busulfan is an alkylating chemotherapeutic agent that is routinely prescribed for leukemic patients to induce myeloablation. However, it also results in azoospermia and infertility in cancer survivors. This research was constructed to explore the possible therapeutic role of amniotic fluid-derived stem cells (AFSCs) in improving busulfan-induced azoospermia in adult rats.
Over the past half a century, survival rates from cancer have improved dramatically. These high cure rates are associated with several long-term sequelae of cancer and its treatment. Unfortunately, male infertility is a major one of chemotherapy drawbacks, and the preservation of cancer patient’s fertility has become a critical healthcare issue.
Busulfan is a common alkylating chemotherapeutic drug. It is used as a myeloablative agent in the conditioning regimens before hematopoietic cell transplantation for patients suffering from chronic myeloid leukemia. However, it exhibits severe adverse effects on all other cells characterized by a physiological high proliferation rate, including the testicular germ cells, up to their eradication and induction of azoospermia.
Stem cells seem to dominate the next frontier of regenerative medicine, owing to their indefinite self-renewal, potential to differentiate into other types of cells, and the fact that, their endogenous repair mechanisms already depend on stem cells residing in different tissues.
Recently, amniotic fluid-derived stem cells (AFSCs) have gained a lot of attention to be used as cell therapy. They display the whole criteria of the adult mesenchymal stem cells (MSCs). Additionally, they harbor a subpopulation that expresses several pluripotency markers, just as the embryonic stem cells (ESCs). Subsequently, AFSCs represent a new class of stem cells with an intermediate phenotype, residing in-between the adult and the ESCs. They could differentiate into cells belonging to the three germ layers without being tumorigenic. These advantageous characteristics, together with the absence of ethical issues concerning their obtainment, have highlighted their potential role in regenerative medicine.
In the present study, they aimed at assessing the histopathological changes of the seminiferous tubules of adult male albino rats, following induction of azoospermia by busulfan. Furthermore, exploring the presence of a possible therapeutic role of AFSCs in improving busulfan-induced azoospermia1.
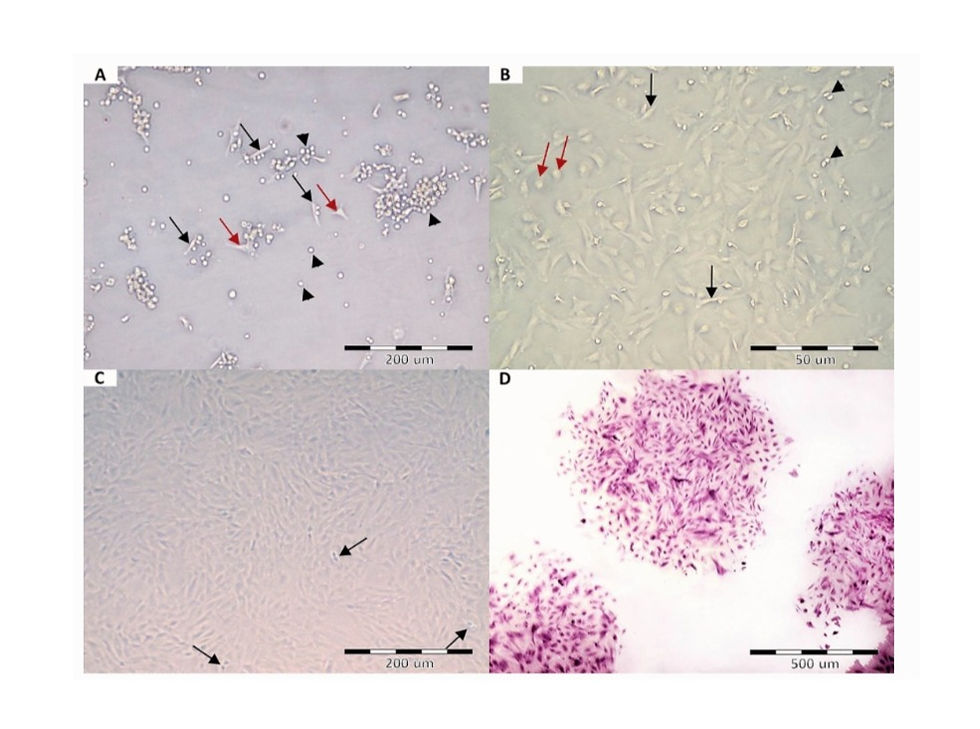
A Passage zero AFSCs after 72 h of cultivation, showing some adherent cells, with either spindle (black arrows) or flat polygonal (red arrows) shapes. The majority of the cells are still not attached to the culture flask (arrowheads). B Passage zero after culturing for 5 days, showing dispersed fibroblast-like adherent cells (black arrows), small rounded floating cells (red arrows), and mitotic cells (arrowheads). C Passage 3 at 80–90% confluency. Arrows; mitotic cells. D Crystal violet-stained colonies

The FACS analysis of passage 3 AFSCs demonstrated that about 35.65% of the cells were positive for the pluripotency marker Oct-4 (Fig. 2A), about 85.12% of the cells expressed the mesenchymal marker CD90, and only about 5.58% of the cells were positive for the hematopoietic marker CD45
AFSCs successfully homed over the basement membrane of the injured seminiferous tubules. They greatly attenuated busulfan-induced degenerative and oxidative changes. They also caused a re-expression of PCNA in the germ cells, leading to the resumption of spermatogenesis and re-appearance of spermatozoa.
Ibrahim, H.F., Safwat, S.H., Zeitoun, T.M. et al. The Therapeutic Potential of Amniotic Fluid-Derived Stem Cells on Busulfan-Induced Azoospermia in Adult Rats. Tissue Eng Regen Med (2021). https://doi.org/10.1007/s13770-020-00309-w






Comments